Impurity challenges in drug development
Controlling impurity is a crucial part of an overall safety strategy in drug development. Potential impurities, whether organic or inorganic, must be identified and categorized in the early stages of drug discovery. And to minimize the level of impurities, appropriate measures are taken at all stages of production, including the selection of raw materials, manufacturing processes, storage conditions, etc.

Currently, for any impurity that appears during the synthesis process, chemists must manually analyze and identify its structure in order to select appropriate reaction conditions to prevent or reduce formation of impurities. Identifying impurities requires extensive experience in synthesis. Researchers have to spend a lot of time retrieving literature and conducting complex compound purification before arriving at a conclusion. The whole process can be exceedingly time-consuming and labor-intensive, so there is a need for an efficient method of impurity analysis to expedite drug discovery.
Artificial intelligence helps predict impurities
As artificial intelligence (AI) has advanced significantly and its use spans various industries, Chemical.AI has successfully developed multiple proprietary solutions to accelerate the digitalization of drug R&D. An AI-powered retrosynthesis routes design platform (ChemAIRS) , a intelligent lab management system (ChemAIOS), and an AI and IoT solutions for chemical laboratories (ChemAIoT), are designed to free up scientists to focus on creative thinking. These solutions not only help build a next-generation data ecosystem, but also enhances efficiency of drug discovery. One specific approach involves overcoming the challenge of impurities, which is why ChemAIRS released a new feature called Impurity Prediction.
In terms of predicting impurities in organic chemical reactions like metal-catalyzed dehalogenation, over oxidation or reduction, metal-catalyzed self-coupling, side reactions in hydrolysis and deprotection reactions, and regioselective by-products, ChemAIRS has reached the level of a chemist with extensive expertise. It has successfully predicted impurities in common organic reactions, in particular, reagent (e.g., HATU, DCC, etc.) metabolites with accuracy above 90%.
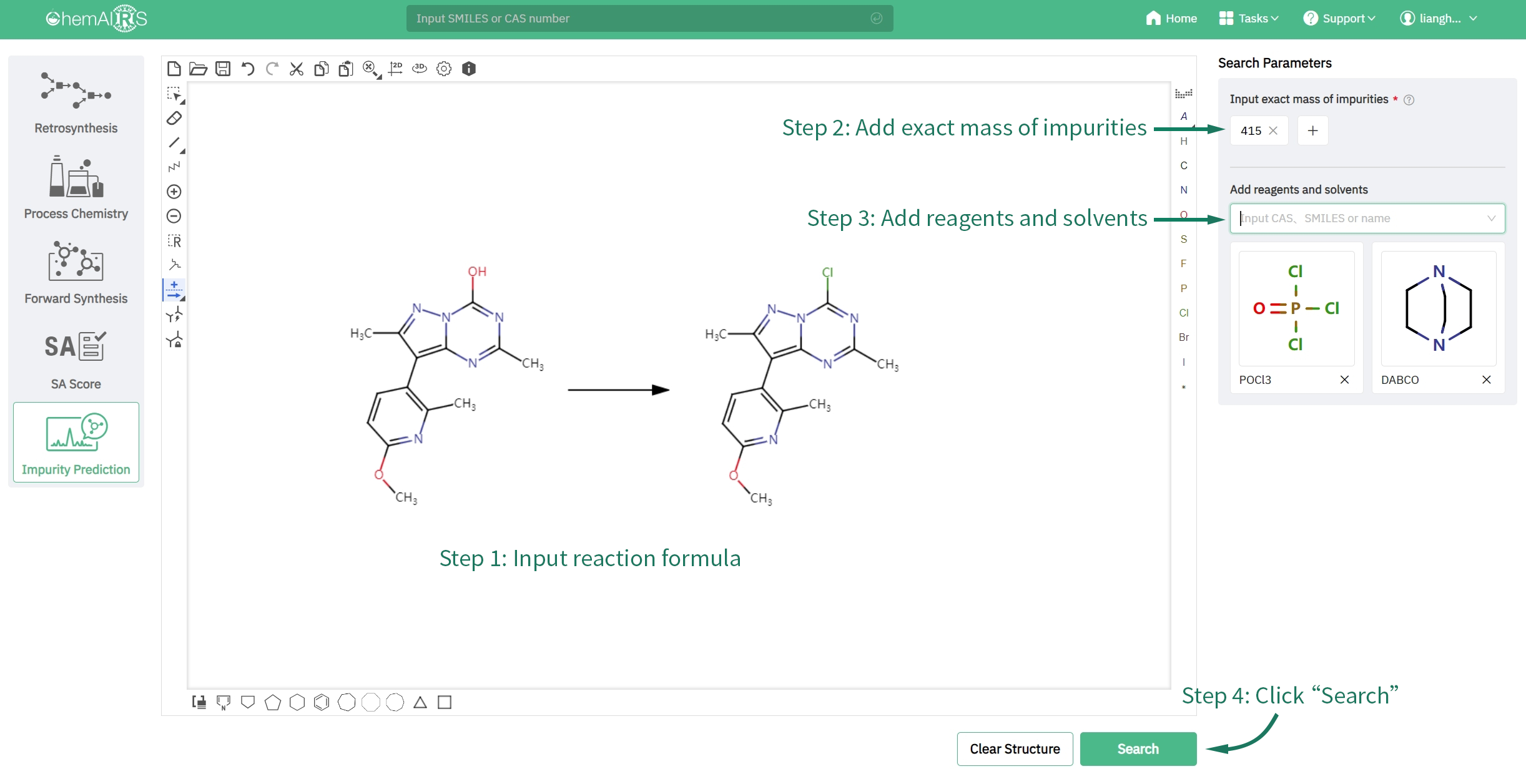
How to make a prediction with ChemAIRS
Input reaction formula → Input molecular weight of impurities → Add reagents and solvents → Click “Search” button to find out the impurity structure predicted.
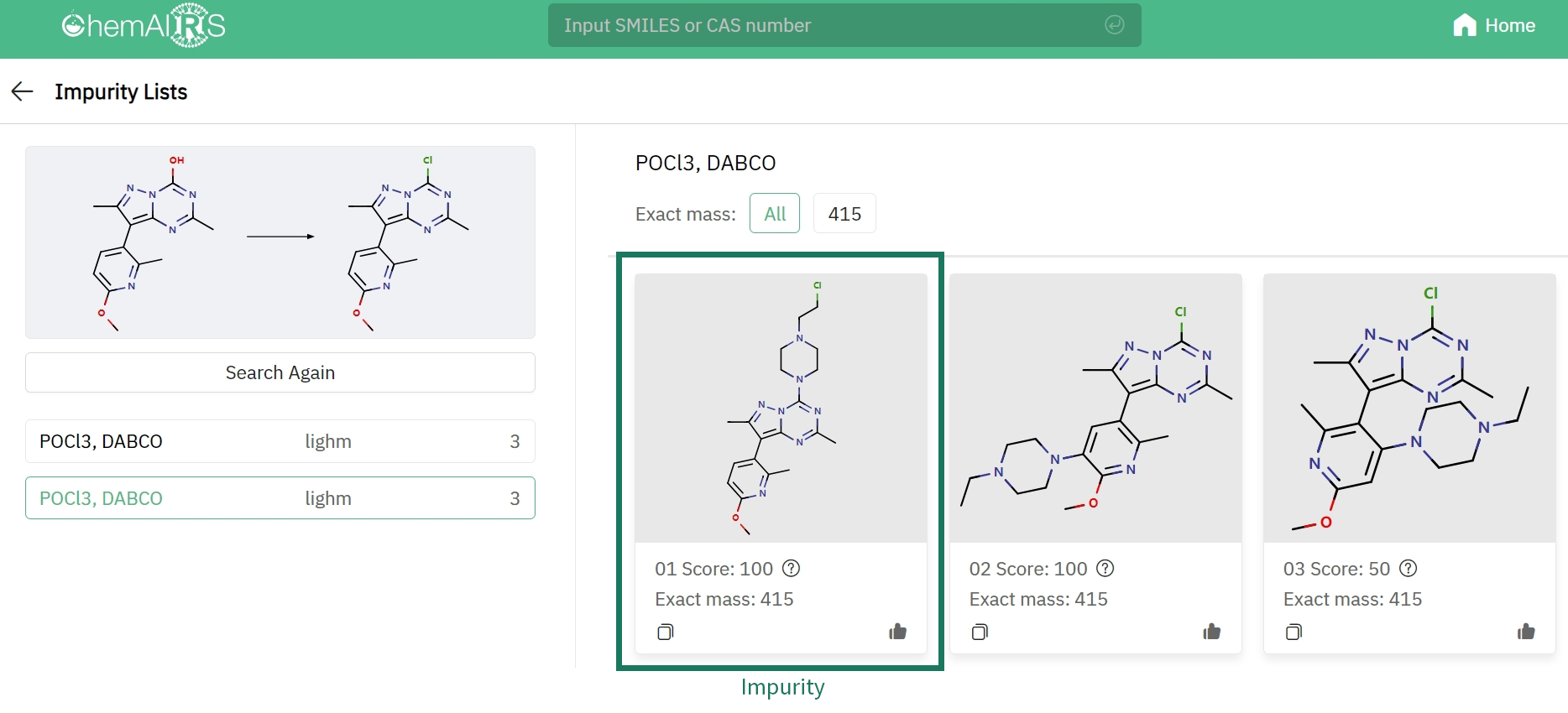
Impurity prediction examples
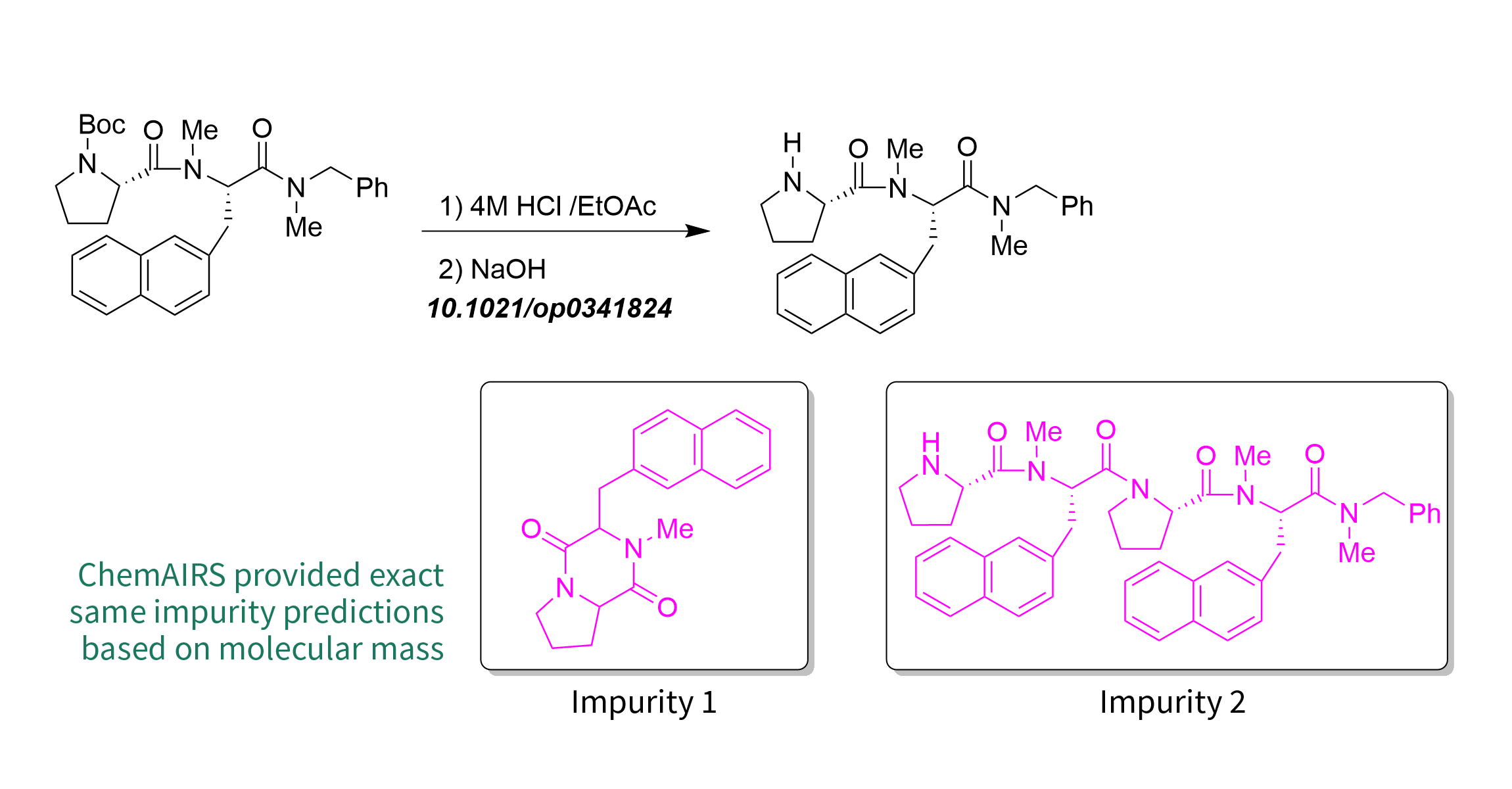
Case 1
To evaluate the effectiveness of the ChemAIRS impurity prediction model, we selected a literature from Organic Process Research & Development (ORPD) which discussed the synthetic process and impurity studies of the tachykinin receptor antagonist TKA731 [1]. One of the synthetic processes was BOC group deprotection, which gave satisfactory yield only on a small scale. With a larger reaction scale, the yield reduced significantly to 50%. When further addressing the impurities, researchers found that the nitrogen atoms in the deprotected pyrrolidine attacked its amide carbonyl terminal under acidic conditions, resulting in impurity 1 formed by intramolecular cyclization and impurity 2 formed by intermolecular coupling. Structure elucidation of these types of impurities often requires purification and further characterization like NMR. Nevertheless, with AI algorithms in ChemAIRS, potential impurity structures and formation routes are provided within minutes, saving valuable research times.
Case 2
Another paper from the OPRD journal studied the synthesis process of pyrazolotriazine, corticotropin-releasing factor receptor 1 antagonist (CRF1) [2]. Two types of impurities were identified in the development of this process. Impurity 1 was formed through a substitution reaction between the reagents and products, which can usually be deduced from its molecular mass. However, inference of impurity 2 could be challenging even for experts. Separation (purification) and NMR are often utilized to ensure an accurate identification in such case. Researchers hypothesized that the formation of impurity 2 was the result of nucleophilic attack of DABCO on the O-phosphorylated intermediate, followed by DABCO ammonium structure opening by Cl(-). ChemAIRS successfully found both impurities in few minutes. The result suggests that ChemAIRS is able to derive impurities of certain structural complexity with explainable mechanisms proved by chemists.

Case 3
We tested against a reaction in a patented synthesis of CDK2 inhibitor [3]. In the synthesis of target compounds containing 1,1-Difluoroethyl group, scientists noticed that half of the yields are impurities. They believed this was mainly due to the fact that fluorine atoms are also highly electronegative and the target compounds tend to be eliminated and form mono-fluorinated alkenes under alkaline conditions. Only a small fraction of synthetic chemists is familiar with fluoroorganic reactions and their mechanisms, leading to some difficulties in corresponding impurity identifications. ChemAIRS, once again, accurately predicted the impurity structure in this study, demonstrating its capacity of compensating for the lack of experience.
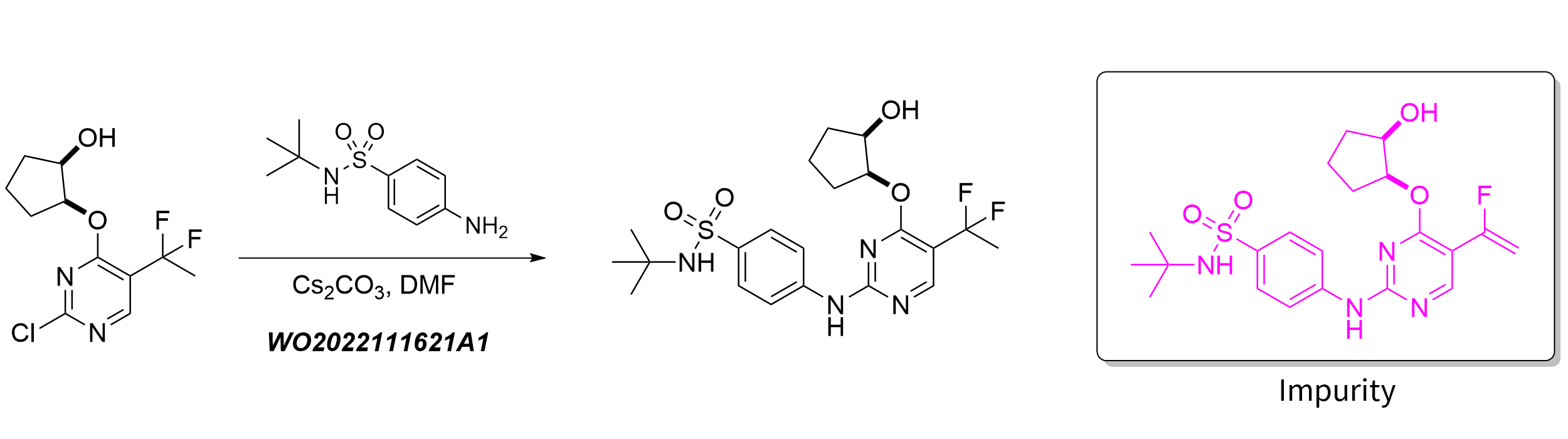
From the three cases presented above, it is clear that ChemAIRS offers both remarkable speed and high accuracy when predicting reaction impurities. It can assist chemists in analyzing experimental results and compensate for any lack of human experience. ChemAIRS excels in replicating chemists' impurity analysis work thanks to the utilization of Chemical.AI's proprietary AI algorithms. It not only generates synthesis routes and explores reaction conditions but also empowers impurity identification in drug development and chemical process optimization. The impurity prediction feature improves as our AI algorithm continues to advance.
In addition to online prediction, ChemAIRS could also be deployed locally. In this way, electronic lab notebook (ELN) and analytical instruments (such as LCMS) are merged to create an in-house impurity prediction ecosystem. It enables the recording of each ELN and allows effective AI learning of any related impurity data. This is a remarkable step for the digital intelligence of pharmaceutical research as it accelerates the process of creating the next-generation digital lab ecosystem.
Reference
[1] Prashad,M. et al, Process Development of a Large-Scale Synthesis of TKA731: A Tachykinin Receptor Antagonist. Org. Process Res. Dev. 2004, 8, 330.
[2] Broxer,S. et al, The Development of a Robust Process for a CRF1 Receptor Antagonist. Org. Process Res. Dev. 2011, 15, 343.
[3] Cheng, D. et al, Aminoheteroaryl Kinase Inhibitors. WO2022111621A1.




